Epilepsy affects more than 65 million people worldwide. Genetics plays a key role in understanding and managing the disorder, and genetic testing has become a powerful diagnostic tool — studies show that up to 75% of patients experience improved outcomes after receiving a genetic diagnosis.
For clinical genomics and R&D teams, the issue isn’t a lack of data — it’s navigating the abundance of it. Evidence is fragmented across studies, variant databases, and publications, making it hard to distinguish between well-established associations and those emerging from research.
The Challenge: Too Much Data, Too Little Connection
DISGENET helps close this gap by connecting research and clinical data into one verifiable source. It integrates evidence, genes, and associations from across studies. As a result, scientists, clinicians, and bioinformatics teams can explore epilepsy genetics with confidence and clarity. With more than 93% average coverage across major epilepsy panels, DISGENET provides a consistent foundation for both discovery and diagnostics.
Curious how your team could streamline evidence tracking? Book a demo of DISGENET to see how it works.
What 10 Epilepsy Gene Panels Reveal with DISGENET
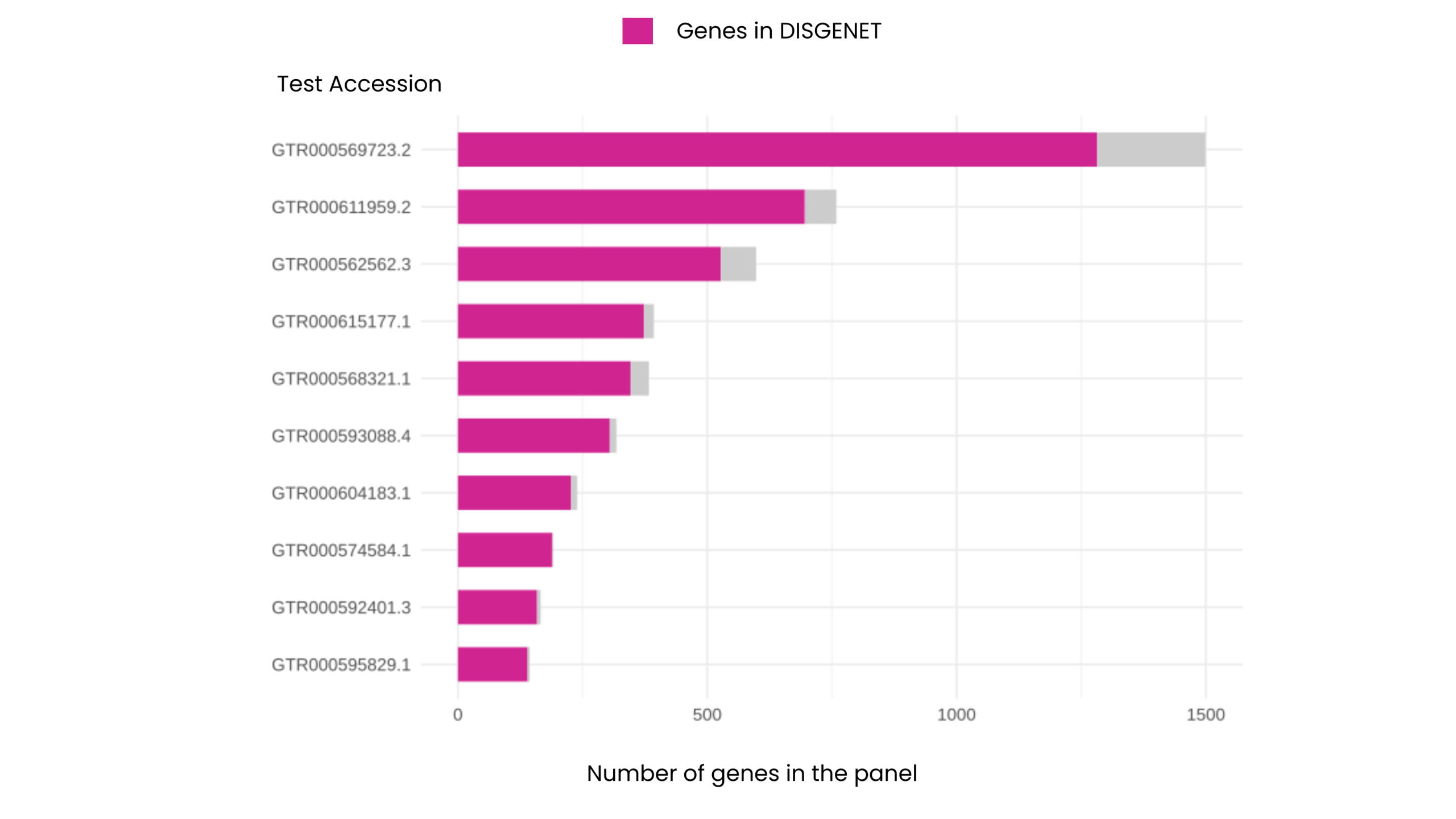
We compared 10 epilepsy gene panels from top clinical genomics labs — each with at least 100 genes and one panel per lab. One trend became clear: no two panels were the same. Panel sizes ranged from 144 to nearly 1,500 genes, together covering over 1,800 unique epilepsy-associated genes. This diversity highlights how differently laboratories interpret epilepsy — and why a unified evidence source is critical.
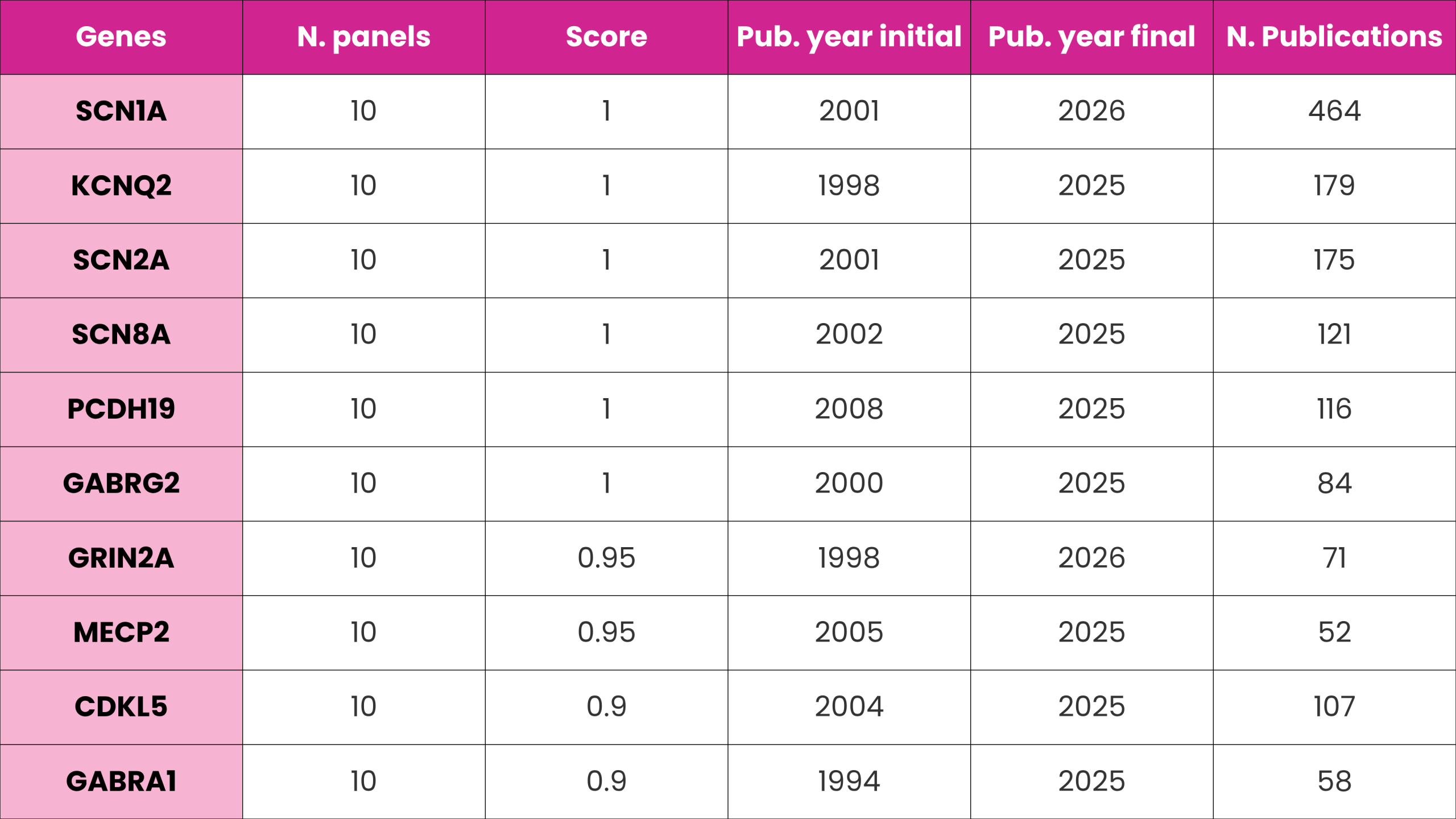
Across all 10 panels, certain genes, including SCN1A, KCNQ2, SCN2A, SCN8A, and GABRA1, appear most frequently, reflecting their central role in epilepsy genetics and forming the foundation of clinical testing worldwide. This pattern is mirrored in DISGENET, where these genes show strong association scores, high publication counts, and sustained research interest over time.
From Fragmented Evidence to a Single Source
On average, DISGENET includes 93% of the genes found in each epilepsy panel.
DISGENET’s extensive coverage shows its strength as a comprehensive, evidence-backed platform. It also enables clinicians and researchers to move confidently from a gene list to a clear understanding of biological relevance. Therefore, users can explore evidence strength in one place — without needing to check multiple databases.
Search epilepsy panel genes in DISGENET and explore their evidence profiles.
How DISGENET Makes Epilepsy Gene Discovery Simple
New Opportunities in Epilepsy Genetics
Once panel coverage is established, the next question for research and clinical teams is clear: what lies beyond today’s diagnostic boundaries?
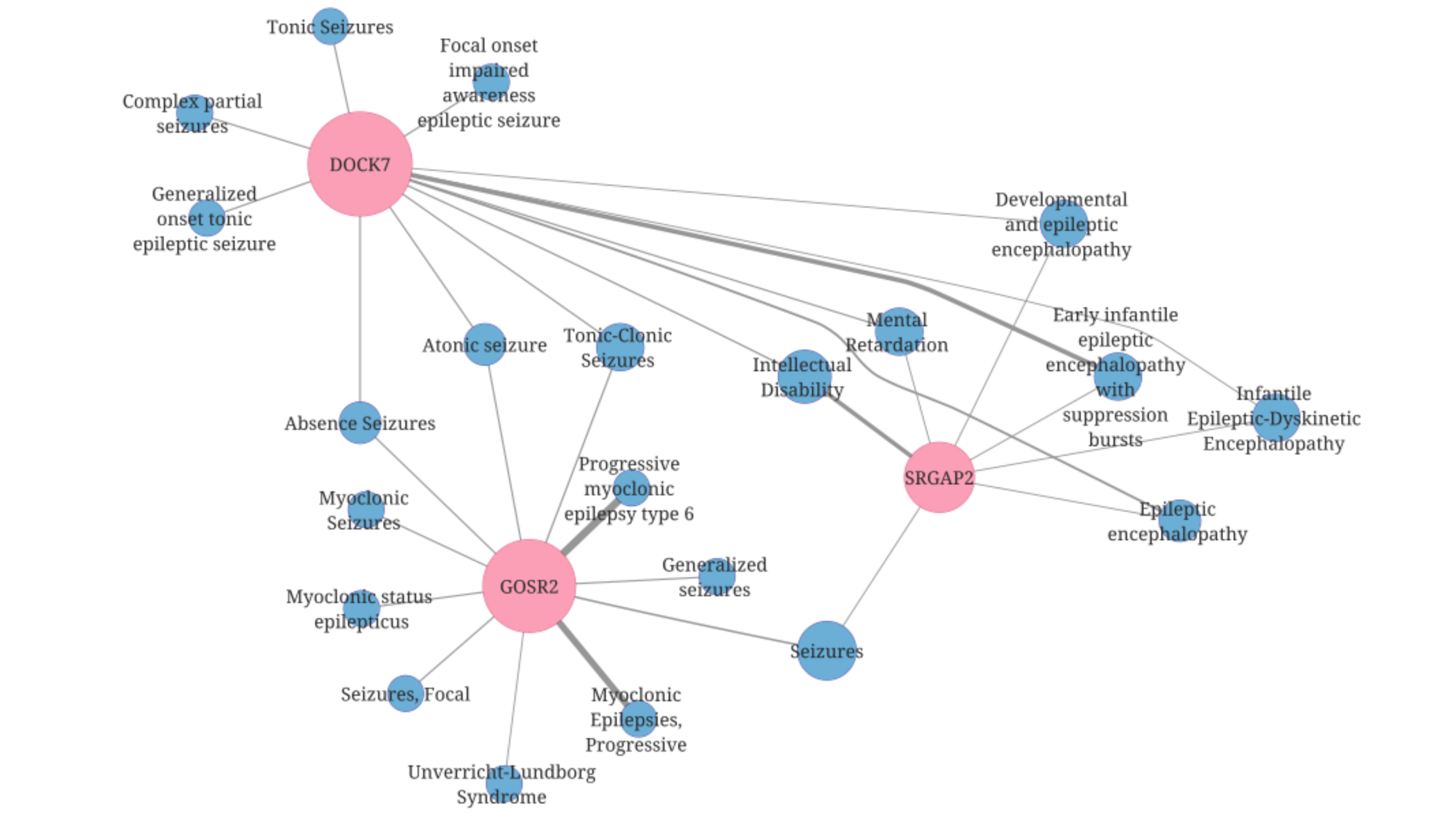
The DISGENET analysis uncovered over 2,000 additional epilepsy-associated genes not yet consistently included in clinical panels. This finding highlights new opportunities for discovery and innovation. Pre-clinical and clinical studies support many of these genes, suggesting emerging candidates that teams can now monitor and explore.
Why Some Genes Differ Between Panels and DISGENET
Among these additional genes, several — such as ABCB1, AQP4, SLC2A8, and GABRG1 — already show strong experimental evidence yet are not included in any of the panels analyzed. Furthermore, the data shows that these genes are associated with specific epilepsy subtypes and related conditions, supporting the development of more precise genetic tests.
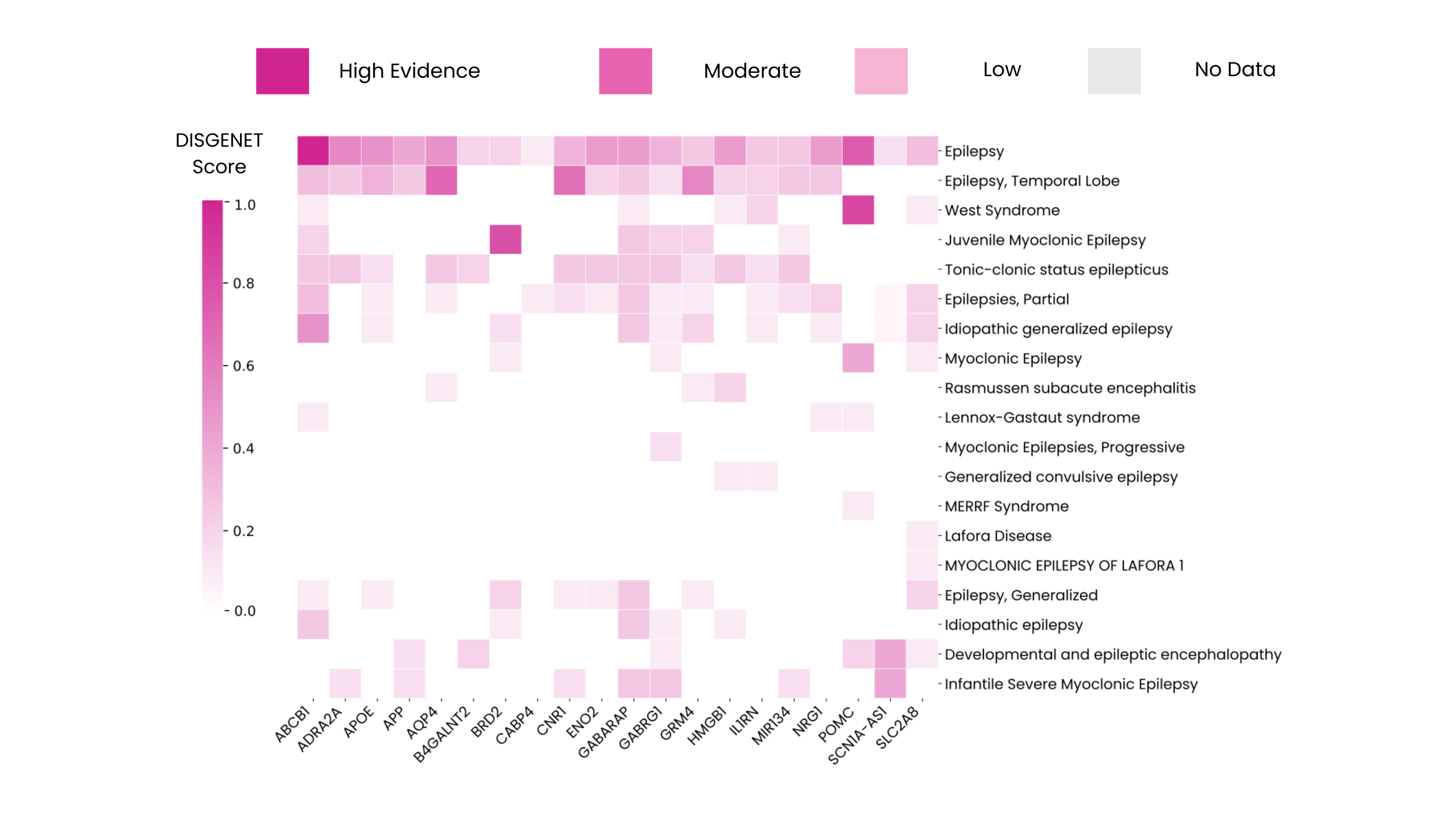
Although some genes included in clinical panels are not yet annotated in DISGENET as epilepsy-associated, every gene from the analyzed panels is represented in the platform. This gap reflects how diagnostic testing often takes a broader or anticipatory view — including genes linked to related syndromes or overlapping neurological traits. Nevertheless, the few genes not directly linked to epilepsy are associated with related nervous system conditions such as intellectual disability, mental retardation, and phenotypes such as seizures.
Clinical and Research Applications
• For clinical genomics teams: faster validation of epilepsy-associated genes and stronger benchmarking against published data. This, in turn, helps laboratories refine diagnostic panels, confirm variant pathogenicity, and improve patient stratification.
•For biotech and pharmaceutical R&D: It accelerates the discovery of novel therapeutic targets and candidate biomarkers linked to specific epilepsy subtypes. These insights support data-driven validation studies that can extend beyond current diagnostic and treatment boundaries.
Overall, DISGENET acts as a bridge between validated and exploratory genomics, revealing how new associations in epilepsy genetics can emerge before they reach clinical adoption.
Explore epilepsy data in DISGENET
Advancing Precision Neurology Together
Epilepsy research stands at the crossroads of clinical genomics and data-driven discovery. As part of Epilepsy Awareness Day, we highlight the progress — and the data — driving better diagnostics and more connected research.
DISGENET brings together nearly 2,000 panel genes and 2,000+ additional discovery genes. Through this integration, scientists connect knowledge, close gaps, and move closer to precision medicine in epilepsy.
Key Takeaways
- 93% coverage of epilepsy panels in DISGENET
- 10 epilepsy panels, more than 1,800 epilepsy genes
- Over 2,000 additional epilepsy genes identified in DISGENET
- Designed for clinical genomics, bioinformatics, and biotech R&D
- Evidence-based, integrated, and ready for exploration
